May the intermolecular forces be with you!
Your students can compare and contrast different types of intermolecular forces with this innovative model. First students make model atoms and electrons so they can look at electronegativity in covalent bonds.
Download and print as many times as you want - forever at no extra cost!
They create 3 dimensional models of atoms that have electrons orbiting the nucleus to see how van der Waals forces happen.
The students next look at permanent dipole-permanent dipole interactions by looking at what makes molecules polar or non-polar.
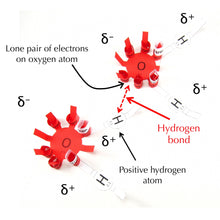
Finally they look at how hydrogen bonds are formed.
Includes full colour Powerpoint class presentation!
Takes about 30 minutes! Model atom diameter 10cm/4in
Download includes: instructions, model templates, and Powerpoint presentation.
All you need: paper, scissors and tape